Abstract
Background: The PD-1 inhibitors release T cells from PD-L1 and PD-L2 mediated blockade, resulting in enhancement of immune responses. These immune responses include not only antitumor activity but also autoimmune reactions. The most serious toxicities of PD-1 inhibitors are immune-mediated, consistent with their mechanism of action. The purpose of this retrospective analysis was to characterize the immune-related adverse reactions (IMARs) of PD-1 inhibitor therapy in patients with cHL.
Methods: Data were pooled from three single-arm trials in adults with multiply-relapsed or refractory cHL treated with nivolumab or pembrolizumab at their approved doses. The pool included 243 patients from Study CA209-205 (NCT02181738), 23 patients from Study CA209-039 (NCT01592370), and 210 patients from Study MK-3475-087 (NCT02453594). IMARs were ascertained using grouped MedDRA terms for the following categories: adrenal insufficiency, cardiac disorder, diabetes, diarrhea, encephalitis, hepatitis, hypophysitis, infusion reaction, kidney injury, myositis, pancreatitis, pneumonitis, rash, thyroid disorder, and uveitis.
The pooled data set was queried for the incidence, timing, and outcome of IMARs in the population overall. Key patient characteristics assessed as risk factors for IMARs included age, sex, race, ethnicity, weight, body mass index (BMI), B symptoms, bulky disease, prior irradiation, prior autologous stem cell transplantation (aHSCT), prior brentuximab use, and prior lines of therapy. To identify risk factors, multivariable models were developed using a backward-stepping elimination procedure after the initial selection using p-value < 0.2 in the univariable analysis. In the multivariable analysis, p-values < 0.05 were considered statistically significant.
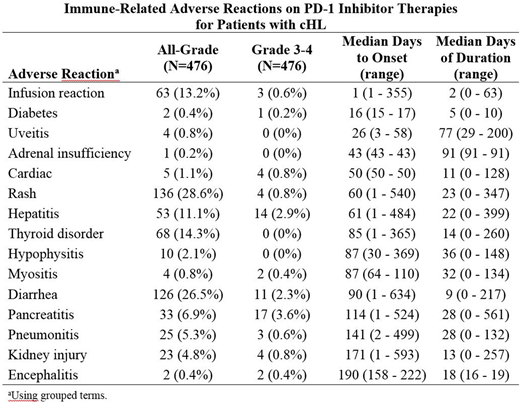
Results: The study cohort included 476 patients of median age 34 years (range, 18-76 years); 56% were male, and 87% were Caucasian. Treatment consisted of a median of 13 doses over a median of 9.3 months. An IMAR was reported in 301 (63.2%) patients, and the IMAR was grade 3-4 in 52 (10.9%) patients. No fatal IMARs were reported. There were 51 (11%) patients treated with corticosteroids, and 48 (10%) had study drug interrupted or withdrawn. The most common (>10%) IMARs were rash (28.6%), diarrhea (26.5%), thyroid disorder (14.3%), infusion reaction (13.2%), and hepatitis (11.1%). The most common (>1%) grade 3-4 IMARs were pancreatitis (3.6%), hepatitis (2.9%), and diarrhea (2.3%). The table below shows the IMARs listed in order of time to onset. Risk factors for the occurrence of any IMAR were prior aHSCT (p=0.004) and elevated BMI (p=0.049). When assessed by individual IMAR, the risk factors identified were age for cardiac disorder; prior aHSCT for diarrhea; race for hepatitis; race and weight for infusion reaction; number of prior lines of therapy and BMI for kidney injury; weight and B symptoms for pneumonitis; prior aHSCT and BMI for rash; and sex and prior radiation for thyroid disorders. No risk factors were identified for adrenal insufficiency, diabetes, hypophysitis, myositis, pancreatitis, or uveitis.
Conclusions: The majority of patients with cHL treated with a PD-1 inhibitor experienced an adverse event consistent with an IMAR, although few events were grade 3-4. Most of these events started after 2-3 months of treatment, but overall, they occurred at any time during treatment. Prior aHSCT was identified as a significant risk factor for IMAR, although this was driven largely by the categories rash and diarrhea.
Acknowledgment: Data were provided for nivolumab by Bristol-Myers Squibb Company and for pembrolizumab by Merck Sharp & Dohme Corp.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.